Abstract
Therapeutic plasma exchange (TPE) is increasingly utilized for Grade 3-4 immunotherapy adverse events (irAEs) to accelerate elimination of drug, autoantibodies, and cytokines. Immune checkpoint inhibitors (ICI) are IgG1 or IgG4 constructs with long half-lives (t1/2) and volume of distributions (Vd) favorable for clearance by apheresis. As drug levels are not readily available, the number of sessions needed to eliminate ICIs is estimated based on the interval from last drug dose, drug Vd, and drug t1/2. Increased use of TPE is anticipated as ICIs are now given despite preexisting autoimmunity, prior organ transplantation, and/or prior irAEs. TPE protocols are well established for the management of diseases that can also present as irAEs [e.g. thrombotic thrombocytopenic purpura (TTP), chronic inflammatory demyelinating polyneuropathy (CIDP), myasthenia gravis (MG), hemophagocytic lymphohistiocytosis (HLH)] and for drug removal (e.g. natalizumab). While TPE is included in irAE management guidelines as second line therapy or later (Brahmer JR, et al, J Immunother Cancer, 2021 PMID: 34172516), no randomized controlled trial (RCT) data are available. TPE can reduce the level of clinical biomarkers for irAE monitoring: creatinine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT),fibrinogen, platelet count, ferritin.
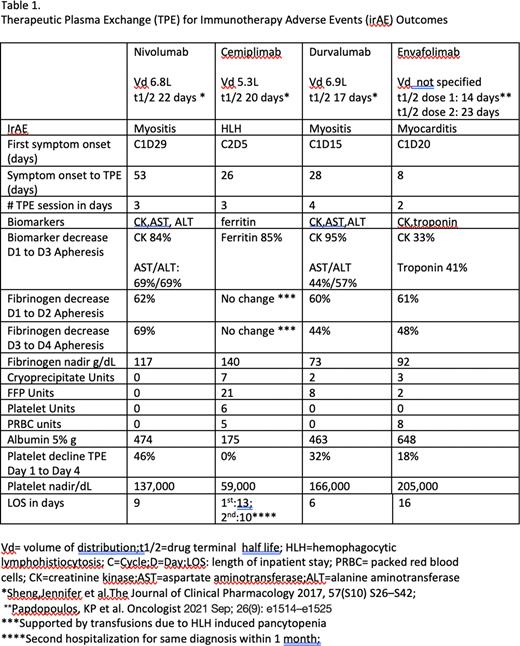
We treated 4 adults (median age 66 years, range 65-77 years) with TPE for steroid-refractory irAEs [myositis (2), myocarditis (1)] and, as part of initial therapy, in combination with dexamethasone and anakinra, for ICI-associated HLH. ICI constructs were IgG1 anti-PD-L1 (envafolimab, durvalumab) and IgG4 anti-PD-1 (nivolumab, cemiplimab). Data collection was IRB approved and with patient written consent. ICI indications were sarcoma, thymic carcinoma, cutaneous squamous cell carcinoma (SCC), and lung SCC. Only one patient (HLH irAE) had preexisting autoimmunity (thyroiditis). Median time from last ICI dose to first irAE symptoms or signs was 16 days (range 5-29 days). Three of 4 patients developed irAE after the first ICI dose. One patient (myocarditis) required full systemic anticoagulation (heparin, then enoxaparin) for atrial fibrillation and deep venous thrombosis, and dual antiplatelet therapy (DAPT) for a new coronary stent. The two myositis patients received prophylactic enoxaparin.
All TPE sessions were one plasma volume exchange through central venous access, utilized Anticoagulant Citrate Dextrose Solution A (ACDA), and scheduled daily or every other day. Number of sessions ranged from 2-4. Except for the patient with HLH, TPE was initiated with 5% albumin replacement. Fibrinogen levels and estimated bleeding risk guided the need for fresh frozen plasma (FFP) replacement. Session intervals, exchange fluid, transfusion, biomarkers, changes in fibrinogen and platelet count, anticoagulation, and functional response are detailed in Table 1. Three of 4 patients, who had normal baseline fibrinogen and platelet counts, experienced clinically significant hypofibrinogenemia resulting in discontinuation of chemical DVT prophylaxis and/or cryoprecipitate transfusion, but did not have clinically significant thrombocytopenia. The fourth patient (HLH) required ongoing transfusion support due to pre-TPE hypofibrinogenemia and thrombocytopenia. TPE confounded interpretation of biomarkers in all. All irAEs demonstrated prompt clinical improvement, however, due to rapid corticosteroid tapers, 2 patients (myositis, HLH) relapsed within 1 month from last TPE. Both responded to additional immunosuppression. One patient (myositis) did not relapse. Grade 4 bleeding occurred in the patient receiving enoxaparin and DAPT and led to discontinuation of TPE after 2 sessions.
Our experience supports the following when considering TPE for irAEs: 1) exclusion of patients with high bleeding risk, or use of 100% FFP replacement and every other day TPE; 2) meticulous non-biomarker irAE assessment during and after TPE; 3) RCT design includes irAE relapse timing and rates and long term follow-up to assess cumulative immunosuppression and tumor outcome; 4) serial drug levels and correlative studies (e.g. cytokines, autoantibodies) to better understand TPE mechanisms, persistence of irAEs, and long term ICI anti-tumor benefit.
Disclosures
Muppidi:Ra Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Agenx: Membership on an entity's Board of Directors or advisory committees; Horizon Therapeutics: Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Katsumoto:Sanofi SM: Research Funding; Genentech: Consultancy; Sonoma Biotherapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal